Discovery of [Fe]-hydrogenase in bacteria opens new possibilities for conversion of hydrogen
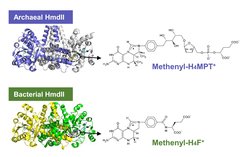
Structures of HmdIIs from Bacteria and Archaea and their substrates.
[Fe]-hydrogenase (Hmd) catalyzes H2-activation and hydrogenation reactions using a unique prosthetic group, the FeGP cofactor. This enzyme operates in methanogenic archaea and uses the methanogenic C1-carrier tetrahydromethanopterin (H4MPT) in form of methenyl-H4MPT+ as electron acceptor for H2 oxidation. Genomes of a bacterial genus Desulfurobacterium contain genes for the biosynthetic enzymes of the FeGP cofactor and a paralog of [Fe]-hydrogenase (HmdII). This is remarkable because [Fe]-hydrogenase, its paralogue and the FeGP cofactor have never been found in bacteria and the putative substrate of [Fe]-hydrogenase, methenyl-H4MPT+, is absent in this organism. The research by Seigo Shima and colleagues provided experimental evidence for the biosynthesis of the FeGP cofactor in Desulfurobacterium and its utilization by bacterial HmdII. Interestingly, the reconstituted bacterial HmdII catalyzed Hmd reactions with tetrahydrofolate derivatives (the C1-carrier in bacteria). “This discovery has a great potential to impact future biotechnical applications”, Seigo Shima explains, “An Hmd variant implemented into bacteria could provide energy to power C1 metabolism directly from H2”.



![Reconstruction of a central [Fe]-Hydrogenase metallocofactor in a test tube](/1207025/original-1650443086.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjoxMjA3MDI1fQ%3D%3D--1a3b0bc51a772685a00608c9f5e1a7b14a106143)






![<p>Atomic crystal structure of activated [Fe]-hydrogenase</p>](/636742/teaser-1716364267.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjo2MzY3NDJ9--4e67b9a3a27597158206bf7d411649c4c854ef47)
![<p>[Mn]-hydrogenase: A new step towards redesigning hydrogenases</p>](/584881/teaser-1560940570.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjo1ODQ4ODF9--dc69e8f47e0c4c4922871284f94371c72e52b9c9)
