Research Area
In their natural habitats, bacteria mostly grow in highly structured multispecies communities. These range from freely floating aggregates to surface-attached biofilms and host-associated ecosystems. Development, structure and function of such communities is dictated by a range of interactions including intercellular signaling. A common form of such signaling is known as quorum sensing (QS), which is based on the ability of bacteria to produce and synchronically respond to small molecules termed autoinducers (AIs). The substantial repertoire of such molecules, ranging from acyl homoserine lactones in Gram-negative bacteria to small peptides in Gram-positive bacteria allows effective induction of intraspecies communication and group behavior in population density-dependent manner. Furthermore, integration of self- and non-self-produced AIs in a growing multispecies community results in group-wide changes in transcriptional programs and thus behavioral patterns of the involved bacteria. Classical examples of QS-induced phenotypes include bioluminescence, biofilm formation, swarming motility, exoenzyme or virulence factors production.
In contrast to structurally diverse intraspecies QS molecules, autoinducer-2 (AI-2) is the only molecule known to be produced and detected by a variety of both Gram-positive and Gram-negative bacteria, principally allowing interspecies communication.
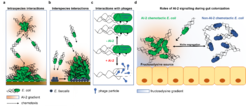
In human commensal Escherichia coli, we showed that chemotaxis towards self-produced AI-2 can mediate collective behaviour-autoaggregation-of E. coli. Such AI-2-dependent autoaggregation enhances bacterial stress resistance and promotes biofilm formation. We further demonstrated that autoaggregation behaviour and biofilm formation by E. coli are enhanced in presence of Enterococcus faecalis that naturally co-occurs with E. coli in the mammalian gut, wound and urinary tract infections. Formation of such mixed dual-species biofilms increases stress resistance of both E. coli and E. faecalis alike. Intriguingly, we could also show that T1 phage of E. coli, previously known as a lytic phage, can enter a pseudolysogeny state and its induction is controlled by AI-2 and metabolic state of the host. These findings clearly show the important role of AI-2 in bacteria-bacteria and bacteria-virus interactions.
AI-2 is a major autoinducer molecule in the mammalian gut, and manipulation of luminal AI-2 concentration was shown to influence the abundance of the major phyla of the gut microbiota, the balance of which in turn can potentially affect human health. Despite the apparently important role of AI-2 signaling in the context of the mammalian gut, its involvement in bacteria-bacteria and bacteria-host interactions remains largely unclear. We showed that chemotaxis provided motile E. coli across different phylogroups with fitness advantage during gut colonization. The benefit of chemotaxis was further largely dependent on bacterial response to self-produced AI-2. E. coli strains across the phylogenetic tree show a certain degree of variability in terms of presence/absence of the lsr operon and thus ability to perform chemotaxis towards AI-2. Such heterogeneity might results in niche segregation and stable co-existence of different E. coli strains in the gut.
Although the role of AI-2 as a QS signal is now widely accepted, the mechanisms of AI-2 perception and its physiological outcome in many bacteria, including clinically relevant ones, are much less studied.
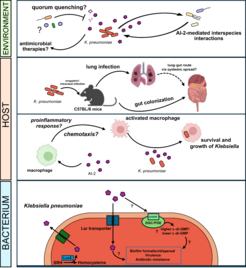
AI-2 signaling in K. pneumoniae cell physiology, virulence and interactions with the innate immune system
Klebsiella pneumoniae an opportunistic human pathogen, is a leading cause of nosocomial infections. It has been widely detected in the intestinal tract, skin and respiratory tract of healthy individuals. Mostly associated with pneumonia, K. pneumoniae also causes urinary tract and wound infections in hospitalized patients. Moreover, rapid emergence of multidrug-resistant strains has been recognized as an urgent threat to human health. K. pneumoniae has thus been a focus of both microbiological and epidemiological studies, and many aspects of its genetics, virulence and transmission routes have been uncovered over the last decades. However, the role of cell-cell communication, specifically AI-2 QS, in host colonization and virulence (including interactions with innate immune cells) is not yet fully understood.
The aim of our work is thus to understand the mechanisms and the roles of AI-2 signaling in K. pneumoniae cell physiology, collective behavior, and host-microbe interactions. As an opportunistic pathogen, it causes lung, urinary tract, and gut infections in humans. Outside of its hosts, it survives and grows in different environments: soil, surface waters. It therefore represents a powerful tool to dissect the principles of AI-2 signaling on different levels, from the molecular level up to host colonization, virulence and interactions with the immune cells. Importantly, a variety of genetic tools are available for this bacterium.
The long-term goal of this research is to understand the principles of interspecies signaling in establishing bacteria-bacteria and bacteria-host interactions, including infection, while advancing our knowledge of the signaling roles of AI-2 at the interdomain interface.
AI-2 signaling outside of the human host and quorum quenching
The increasing presence of K. pneumoniae in environmental sources such as water and soil raises the risk of both animal and human infections. The mechanisms underlying Klebsiella persistence in the environment are not yet fully understood. However, unlike during lung infections, K. pneumoniae interacts with a highly abundant and diverse range of environmental bacterial species. Many of these potential interaction partners, though incapable of producing AI-2 themselves, can still potentially perceive it as a signaling molecule.
Our primary interest is to uncover the mechanisms and ecological outcomes of such signaling, particularly how it influences the persistence and growth of K. pneumoniae in the environment. Furthermore, many environmental bacteria possess a large metabolic repertoire, which includes autoinducer-degrading enzymes that enable them to interfere with the collective behavior of competing microorganisms. Although AI-2-degrading enzymes have not yet been described in environmental isolates, we hypothesize that they exist. By studying natural AI-2 quorum sensing-inhibiting molecules, we aim to lay the foundation for alternative infection treatment strategies beyond antibiotics. This is especially critical in light of the global antibiotic resistance crisis.